Showing posts with label BK virus. Show all posts
Showing posts with label BK virus. Show all posts
Friday, September 17, 2010
Sunday, September 12, 2010
Can BK Virus serum PCR be a good marker for Net immunosuppression?

In the field of transplantation, we are struggling to figure out is the patient on which end of the spectrum, too little immunosuppresion or too much immunosuppression. Markers for rejection have been studied extensively and based on Luminex DSA one can monitor early signs of impending antibody rejection. But many centers are also developing aggressive screening strategies for BK Nephritis. This entity is usually seen as early as 2 weeks post transplant to as late as 7 years post transplant but usually in the first year or so.
I think that A BK serum PCR might be a good marker for NET immunosuppression. Someone who has lupus and has been treated with Cytoxan, Cellcept, Rituxan and has failed kidneys recently and then gets a transplant for the kidney and gets inducted with more immunosuppression might be the highest risk for BK Nephritis much earlier on due to their NET immunosuppresion being the highest. No one can really measure NET immunsuppression. There is a test available called " Cylex" or ImmuKnow . This is the physiology behind it:
"Phytohemagglutinin (PHA) is a non‐specific mitogen which can be used to stimulate cell division in CD4 T‐ lymphocytes regardless of their antigenic specificity or memory status. Therefore, PHA is considered to be a “global” stimulator of the immune system. The production of intracellular ATP is one of the first steps in cellular activation following stimulation with mitogens such as PHA. ATP is a multifunctional nucleotide which plays an indispensible role in the transfer of intracellular chemical energy. The amount of ATP generated can tell us the amount of CD4 T cell activation and the overall immune status of the patient( over or under immunosuppressed). " -from the cylex website( summarized)
But a cell activation can occur in setting on an infection as well and a similar down stream effect on the kidney. Steady monitoring of infectious agents like BKV might be the BEST marker we have to date to tell us " Hey there is too much immunosuppression on board" !
But might not be as simple as that...
Lets see what future studies hold...
Cidofivir for BK Nephritis
 As we all know that treatment for BK Nephritis is tough. The only thing that really works well is Decreasing immunosuppresion. But as we do that, the risk of mounting an immune response arises and you can start seeing biopsies that look like Acute Cellular Rejection and BK staining Positive.
As we all know that treatment for BK Nephritis is tough. The only thing that really works well is Decreasing immunosuppresion. But as we do that, the risk of mounting an immune response arises and you can start seeing biopsies that look like Acute Cellular Rejection and BK staining Positive. At that time, most people will treat with IV Immunoglobulins and see if there is some response in terms of preventing rejection and treating BK at the same time.
So far, no drug has directly targeted BK except Cidofivir. Cidofovir is an antiviral agent that demonstrates in vitro activity against murine polyomavirus and has been proposed for treatment of BKVN in renal allograft recipients. The dose usually recommended is 0.25mg/kg IV every 2 weeks for 8 doses total. This is a low dose and can be used for someone in already some graft dysfunction as cidofivir itself can causing ATN like injury( same as tenofivir). This low-dose cidofovir may be tolerated, even among renal transplant recipients with significant renal dysfunction due to BKVN. Prospective, controlled trials are warranted to further define the optimal dose, toxicity and potential role of cidofovir in renal transplant recipients with BK virus nephropathy.
http://www.ncbi.nlm.nih.gov/pubmed/18380832
http://www.ncbi.nlm.nih.gov/pubmed/16499584
Labels:
BK virus,
Infections,
post transplant complications
Wednesday, August 11, 2010
BKV viral protein-1 mRNA in urinary cells
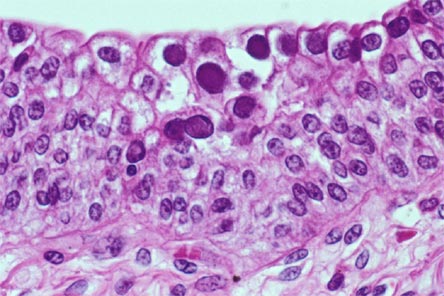
A noninvasive, accurate biomarker for diagnosis of BKVN is being sought. Recent article in Transplantation reports using the urinary cell mRNA profile at the time of BK Nephropathy diagnosis and compared to risk of graft function.
BK Nephropathy was diagnosed with a sensitivity of 100% and specificity of 97% using the urinary mRNA. Levels of granzyme B (GB) mRNA and proteinase inhibitor (PI)-9 mRNA in urinary cells were higher in BKV patients with a subsequent decline in renal function compared with patients with stable function, and were positively associated with rise in serum creatinine from the time of BK diagnosis to 12 months after diagnosis.
This confirms the fact that urinary granzyme B and PI-9 could be used as markers of inflammation during any renal episode post transplant. Similar findings were seen in rejection as well. It seems that no matter what causes the inflammation, BK or rejection, a rise in urinary GB and PI-9 suggests a poor prognosis.
Reference:
Wednesday, July 14, 2010
Leflunomide and liver failure
The FDA just expanded the black box warning on leflunomide( ARAVA), a drug that is sometimes used to treat BK Nephritis in Transplant patients. A recent report showed 49 cases of severe liver toxicity from 2002-2009. Usually the reactions were noted in the first 12 months post use of the drug. The greatest risk occurred in patients taking other drugs that may cause liver damage while taking leflunomide and in patients with preexisting liver disease.
This comes as a shock to some of us in the transplant community as we do use this medication for treatment of BK Nephritis. There is so little in the form of treatment available for BK, this black box warning might make leflunomide not a very user friendly drug anymore.
Subscribe to:
Posts (Atom)






